Antibody production is a false equivalence for clinical protection
vaccine failure after "immunization" and "protective antibody levels"
The tyranny of words. Generally when one hears the word immunity one thinks “being resistant to a particular infectious disease” .
The CDC states:
“Immunity to a disease is achieved through the presence of antibodies to that disease in a person's system”
I will prove this is lacking empirical substantiation.
Patients do not care or know what level of antibodies they have. What is material to them is whether they have a disease with symptoms that negatively impact their lives. There is a fear of disease that is being exploited with vaccination. For example there is extreme fear of paralytic polio despite 95% of cases being asymptomatic. There does not seem to be an equivalent fear of the same paralysis from lead or arsenic poisoning, GBS, transverse myelitis, or vaccine associated paralytic polio (VAPP).
By generating an antibody response does this prove you have sterilizing protection against paralysis? Public health certainly has done its best to indoctrinate this false equivalence. What proof do they have to make these claims?
If you trusted them and got vaccinated and still developed paralysis shortly after would you be satisfied if they could show you generated an antibody response? Of course not. It is the hard clinical end point of preventing paralysis that should be the outcome measure, not antibody production. This clinical benefit should also be demonstrated under real-world conditions without numerous examples of vaccine failure.
Stanley Plotkin is the one of the high priests of vaccines. He authored a paper titled “Correlates of Protection Induced by Vaccination” in which he states:
This review has shown that after the administration of nearly all vaccines, with the exceptions of BCG and zoster, prevention of infection correlates with the induction of specific antibodies. source
Correlate is an interesting word choice. The pro-vaxx mob love the “correlation does not equal causation” argument when a patient is harmed after receiving a vaccine. Lets apply the same standard of evidence to benefits. He defines a correlate as
“an immune response that is responsible for and statistically interrelated with protection”.
Emphasis on responsible for protection. Prove it.
Plotkin provides a chart with his references to make these statements. Lets see if they back up his claims or if he is hoping no one will check.
The first thing on the surface level is that he is frequently only citing 1-3 references. These better be seminal papers essentially proving causation. Spoiler: They are not.
Lets go through the reckless and bloated CDC schedule for the most applicable examples.
1. Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP).
From the package insert:
From the CDC
From 1996 through 2018, 14 cases of respiratory diphtheria were reported to CDC's National Notifiable Diseases Surveillance System (NNDSS), with only 2 cases reported since 2012, in 2014 and 2018. In both instances, disease was caused by non-toxin-producing C. diphtheriae.
So cases were exponentially dropping before any vaccine and the only evidence of benefit is it made antibodies which resulted in the “lowest level giving some degree of protection” or “regarded as protective”. This is deceptive language without any statistical proof as the definition demands. This disease is irrelevant and thus has no clinical indication to be vaccinated against.
It should be noted there is no stand alone injection for tetanus any longer after they closed a production facility. They make you get a combination like DT.
The best evidence they have for tetanus is they generated the lowest protective level of antibodies in 45 mice…This is a mockery. How do people believe this equates to clinical protection against tetanus in humans? It is stated in the medical literature over and over again:
“As vaccinations were believed to be nearly 100% effective in preventing tetanus, tetanus in young, immunized individuals were considered unlikely. However, unexpected tetanus infection has been reported in young adequately immunized individuals.” source
Believed to be nearly 100% effective based on what? The author spreads these indoctrinated beliefs without any citation. They are all under a spell their paycheques do not allow them to reconsider. Admitting they still get “unexpected tetanus infection has been reported in young adequately immunized individuals” is a double bind.
Let’s see what the literature says if people above these levels of protection still got tetanus infection:
“We encountered a case of unexpected tetanus infection in a 20-year-old fully immunized female who made a quick and complete recovery after 6 days in intensive care. This case highlights two important points. Tetanus may still affect the young adequately immunized individuals” source
I have proven my point. Clinical and even fatal tetanus is documented frequently in immunized patients with “protective anti-tetanus titers”. The whole concept is a fraud and they never had the evidence of causality to make the claims of benefit they did.
There are many other treatment options for tetanus including ozone therapy, IV vitamin C, immunoglobulins and antibiotics.
At least they used a clinical end point for preventing pertussis. Too bad the control was a DT vaccine and not an inert placebo. How did it do in the real world?
In “The 112-Year Odyssey of Pertussis and Pertussis Vaccines-Mistakes Made and Implications for the Future” source they state:
Effective diphtheria, tetanus toxoids, whole-cell pertussis (DTwP) vaccines became available in the 1930s, and they were put into routine use in the United States in the 1940s. Their use reduced the average rate of reported pertussis cases from 157 in 100 000 in the prevaccine era to <1 in 100 000 in the 1970s. Because of alleged reactions (encephalopathy and death), several countries discontinued (Sweden) or markedly decreased (United Kingdom, Germany, Japan) use of the vaccine.
Keep this in mind when we learn about polio next. DTP was recalled because of safety concerns and switched to this ineffective acellular one.
The first diphtheria, tetanus, pertussis (DTaP) vaccines were developed in Japan and put into routine use there. Afterward, DTaP vaccines were developed in the Western world, and definitive efficacy trials were carried out in the 1990s. These vaccines were all less reactogenic than DTwP vaccines, and despite the fact that their efficacy was less than that of DTwP vaccines, they were approved in the United States and many other countries. DTaP vaccines replaced DTwP vaccines in the United States in 1997. In the last 13 years, major pertussis epidemics have occurred in the United States, and numerous studies have shown the deficiencies of DTaP vaccines, including the small number of antigens that the vaccines contain and the type of cellular immune response that they elicit. The type of cellular response a predominantly, T2 response results in less efficacy and shorter duration of protection. Because of the small number of antigens (3-5 in DTaP vaccines vs >3000 in DTwP vaccines), linked-epitope suppression occurs. Because of linked-epitope suppression, all children who were primed by DTaP vaccines will be more susceptible to pertussis throughout their lifetimes, and there is no easy way to decrease this increased lifetime susceptibility.
So it does not work. Lets see more examples of it not being an effective immunization.
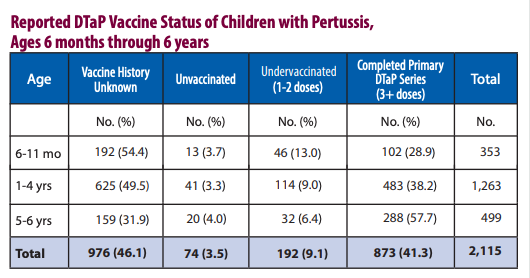
Note they fail to give the percentages of vaccinated in their results and still blame the unvaccinated and incompletely immunized in the conclusion. These people are deplorable.
Great collection of vaccine failure articles shared from Dr. Suneil Jain NMD.
Pertussis circulation cannot be controlled at all. Amazing immunization you got there.
Only 3.5% of pertussis cases were unvaccinated. 41% obeyed the CDC and still contracted the “vaccine preventable disease” after 3 plus “immunizations”. They are hiding a large section of the data as unknown. It is laughable.
From Plotkin himself
From “Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity”
The natural immunity resulting from previous infection is considered more effective than the immunity resulting from vaccinations, and pertussis is no exception
Read that again. I will chose natural immunity over vaccine induced waning failure.
As quoted in Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines
Finally, aPV pertussis vaccines do not prevent colonization. Consequently, they do not reduce the circulation of B. pertussis and do not exert any herd immunity effect. These findings at least partly explain the resurgence of pertussis.
So when someone claims it prevents transmission or generates herd immunity just know they are lying. From the same article
VE was compared after the childhood series (five doses) and after an adolescent booster dose (sixth dose). Relative VE was defined as VE in the population given prior doses of an aPV and absolute VE was defined as VE in an aPV-naïve population. Absolute VE after the childhood series was 91% (95% CI 87–95%) but declined annually by 9.6% (46). Initial relative VE after adolescent boosting was 70% (95% CI: 54 to 86%) and declined by 45.3% annually. The absolute VE of the full six-dose aPV series was estimated to be 85% (95% CI: 84–86%) in the first year after series completion. However, it declined by 11.7% (95% CI: 11.1 to 12.3%) per year, and at 18 years of age, protection was limited to 28.2% of immunized patients (95% CI: 27 to 29%)
What a joke. It does not work. No durable clinical protection. Next.
2. Inactivated Polio Vaccine (IPOL)
I am going to write a separate substack how the this vaccine is likely responsible for the cancer epidemic. Hint one reason is because of the SV40 contaminated monkey kidney cells still used for its production. It also should never have been approved based off a pathetic 3 days safety monitoring. They also assessed safety by co-administrating the whole cell DTP which was recalled because of safety concerns mentioned above. These people are sinister. Read the package insert .
Approximately 90% to 95% of poliovirus infections are asymptomatic... Rapid onset of asymmetric acute flaccid paralysis occurs in 0.1% to 2% of infections, and residual paralytic disease involving motor neurons (paralytic poliomyelitis) occurs in approximately 1 per 1,000 infections.
Prior to the introduction of inactivated poliovirus vaccines in 1955, large outbreaks of poliomyelitis occurred each year in the United States (US). The annual incidence of paralytic disease of 11.4 cases/100,000 population declined to 0.5 cases by the time oral poliovirus vaccine (OPV) was introduced in 1961.
Of the 127 cases of paralytic poliomyelitis reported in the US between 1980 and 1994, six were imported cases (caused by wild polioviruses), two were “indeterminate” cases, and 119 were vaccine associated paralytic poliomyelitis (VAPP) cases associated with the use of live, attenuated oral poliovirus vaccine (OPV). (6) An all IPV schedule was adopted in 1999 to eliminate VAPP cases.
Poliovirus Vaccine Inactivated induces the production of neutralizing antibodies against each type of virus which are related to protective efficacy.
So a mostly asymptomatic disease with a 1 in 1000 risk of paralysis. This vaccine was to replace OPV which was causing paralytic polio..LOL. But it eradicated polio right?
As for the antibodies they are just “related to protective efficacy”. What is the threshold it prevents the only outcome anyone would care about. Paralysis.
Approval in the US was based upon demonstration of immunogenicity and safety in US children. (11)
Would you believe reference 11 is “Unpublished data available from Sanofi Pasteur SA.” These people are crooks.
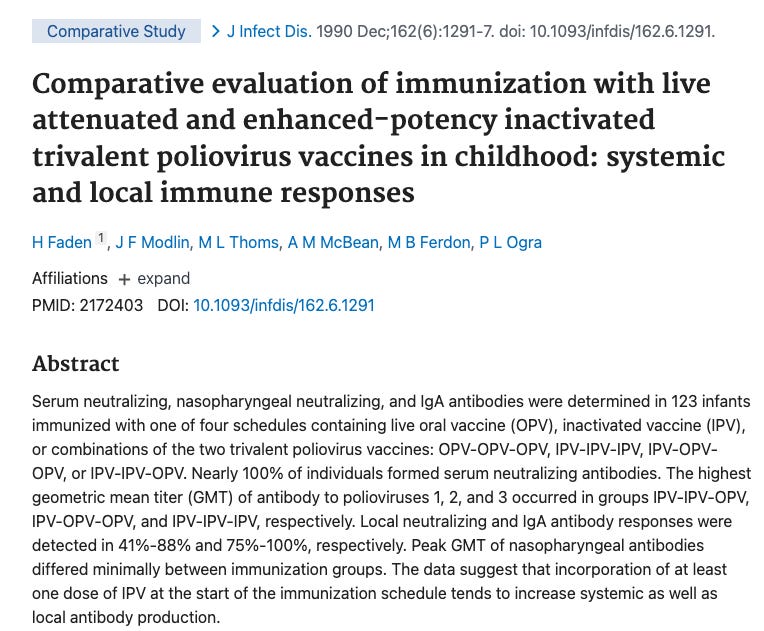
In the US, 219 infants received three doses of a similar enhanced IPV at two, four, and eighteen months of age manufactured by the same process as IPOL vaccine except the cell substrate for IPV was using primary monkey kidney cells. Seroconversion to all three types of poliovirus was demonstrated in 99% of these infants after two doses of vaccine given at 2 and 4 months of age. Following the third dose of vaccine at 18 months of age, neutralizing antibodies were present at a level of ≥1:10 in 99.1% of children to Type 1 and 100% of children to Types 2 and 3 polioviruses.
219 infants is an inadequately powered study. They are citing a “similar enhanced IPV” not the actual product they want to inject you with an use antibodies as their only outcome measure. Lets see how good the science is proving antibodies prevent paralytic polio. Plotkin cites studies 41, 95 and 139.
This is 41. This is study just looks at antibody responses and makes no claim of preventing paralytic polio.
Study 95 does not provide an abstract but from the title it is clear it is not about assessing the level of antibodies that prevent paralytic polio.
Study 139 is a review he is the author of. I find it interesting he starts with his favourite line “The immune system is redundant”. Again there is no proof presented that measuring antibody measurements above a certain threshold will prevent paralytic polio. This whole thing is a fraud and tiresome scam.
So the only benefit they can claim is producing antibodies that in no way provide a clinical benefit. It did not eradicate polio. It did not provide herd immunity. They do not have evidence of any of that. In fact read what UNC Gillings School of Global Public Health has to say about Dr. Bernard Greenberg:
He testified before Congress that statistics had been used inappropriately to determine the effectiveness of the polio vaccine — and that the vaccine actually increased the incidence of polio (1962).
Everything about vaccines you have been indoctrinated to believe is a lie. Maybe you should risk another form of paralysis GBS or death to get “neutralizing antibodies”.
Although no causal relationship between IPOL vaccine and Guillain-Barré Syndrome (GBS) has been established, (28) GBS has been temporally related to administration of another inactivated poliovirus vaccine. Deaths have been reported in temporal association with the administration of IPV (see ADVERSE REACTIONS section)
NEXT.
3. Mumps, Measles, Rubella and Vericella (MMRV)
I have previously discredited the MMR/V shots. This substack documents incredible vaccine failure at high levels of vaccine coverage and shows the lack of hard clinical outcomes in the licensing studies.
Measles vaccination: a proven failure
Measles was in steep decline long before any vaccine was available. Here are the official statistics.
This will destroy the “correlate of protection”.
From “What Is the Evidence to Support a Correlate of Protection for Measles? A Systematic Review”
Yeah. There is no protective titer level of antibodies that mean you cannot get clinical measles. Plotkin cites 24, 120, and 158.
So 24 actually says illness may occur in persons with titers above this level.
Reference 120 which he cites for measles is a study talking exclusively of rotovirus. Careless.
Reference 158 does not show an absolute correlate. Saying seronegative vaccinated children are protected against measles infection disproves the antibodies are what are responsible for clinical protection. These people are shameless.
Is the clinical protection for mumps any better? From “Mumps Outbreak in a Highly Vaccinated University-Affiliated Setting Before and After a Measles-Mumps-Rubella Vaccination Campaign—Iowa, July 2015–May 2016”
Of 453 cases in the county, 301 (66%) occurred in university students. Student cases were primarily undergraduates (90%) and highly vaccinated (86% had 2 MMR doses, and 12% had 3 MMR doses).
98% had ≥2 doses of MMR prior to the onset of illness. This is humiliating. How dare they call this an immunization. Frauds.
Currently, less than 10 people in the US contract rubella each year so I am not going to bother. Just keep in mind CRS used to cause autism and you are injecting a live attenuated version of it.
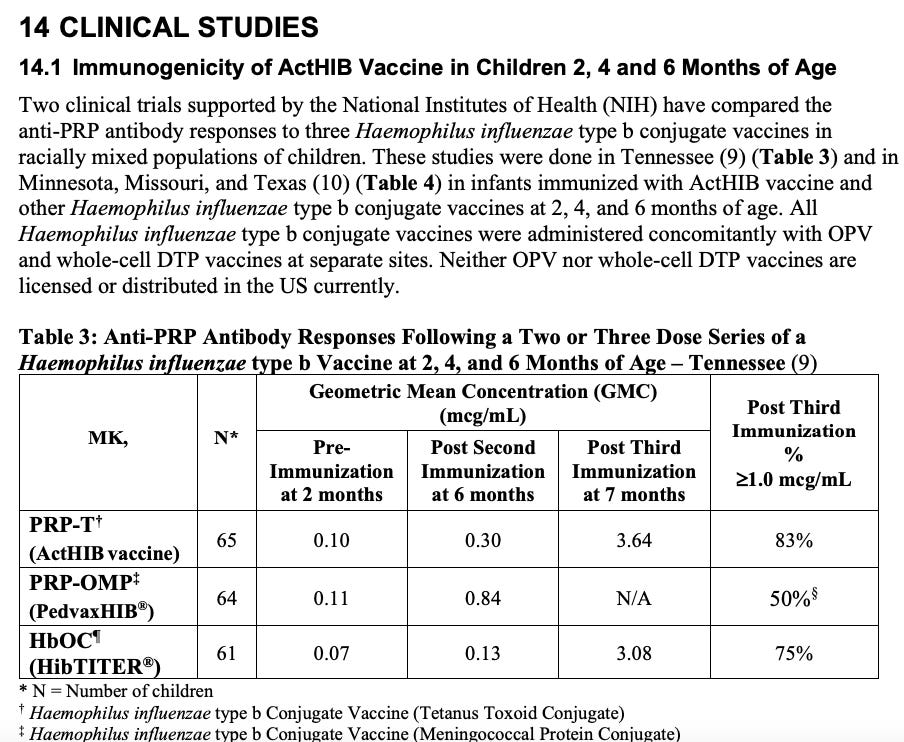
4. ActHIB [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)]
Antibody titers to H. influenzae capsular polysaccharide (anti-PRP) of >1.0 mcg/mL following vaccination with unconjugated PRP vaccine correlated with long-term protection against invasive H. influenzae type b disease in children older than 24 months of age. (7) Although the relevance of this threshold to clinical protection after immunization with conjugate vaccines is not known, particularly in light of the induced, immunologic memory, this level continues to be considered as indicative of long-term protection
Again they administered OPV and DTP at the same time. Neither OPV or DTP are safe for use in North America. This is an unacceptable experimental design.
“the relevance of this threshold to clinical protection after immunization with conjugate vaccines is not known”…
Below is Plotkin’s reference for correlate of protection.
Again does not prove the threshold it prevents clinical infection.
Newborns, on the other hand, are protected by maternal antibodies, and older children and adults possess naturally acquired, protective antibodies. source
So no reason to inject them…
Long-term protection against Hib disease was shown to be associated with PRP antibody levels of ≥0.15 μg/mL in unvaccinated individuals and ≥1.0 μg/mL in children recently vaccinated with purified PRP [12, 13]; such protective levels do not necessarily apply to children immunized with Hib conjugate vaccines, which generate more robust and enduring immune responses. Unfortunately, children under age 18 months, a major risk group, demonstrated poor immunologic responses to the plain PRP vaccine and were not well protected from Hib disease. Although the efficacy of the PRP vaccine was variable in the United States [14], it was licensed for children over age 18 months on the basis of a large study in Finnish children [15]. Considerable controversy surrounded the appropriate use of this vaccine based on regional differences in efficacy and on various interpretations of the data…
Hib invasive infections occasionally occur despite adequate immunization for Hib…
despite a vaccine schedule of 3 doses in the primary series and no booster. After the initial dramatic decrease in Hib invasive disease in South Africa, incidence rates rose from 0.7/100 000 population in 2003 to 1.3/100 000 in 2009; half of the increase represented vaccine failures, which were seen in both HIV-infected and noninfected children
Lots of failure.
Results. Within the first 60 days after disease onset, there was no change in the anti-PRP antibody avidity, and there was no statistically significant difference in the geometric mean Hib antibody avidity over the 3 study periods. However, the children who experienced Hib vaccine failure had significantly lower Hib antibody avidity than did healthy control subjects, despite a marked antibody response following infection.
Conclusions. Children who experience Hib disease despite vaccination appear to have a defect in immunological priming, leading to a qualitative difference in Hib-specific memory B cells. Low anti-PRP antibody avidity decreases the functional activity of anti-PRP antibody in the sera of these children experiencing vaccine failure, leading to disease susceptibility.
and you know it is safe.
In Study P3T06, within 30 days following any of Doses 1-3 of DAPTACEL + IPOL + ActHIB vaccines, 50 of 1,455 (3.4%) participants experienced a serious adverse event (SAE) source
5. Streptococcus pneumoniae (Pneumococcal 15-valent Conjugate Vaccine)
Lack of a definitive serological correlate of protection. No possible way to say antibodies result in clinical protection. What happened in the real world?
the primary study endpoint vaccine efficacy increased with distance from the main study hospital from −14% for children living less than 1.5 km from Bohol Regional Hospital (BRH) to 55% for children living greater than 8.5 km from BRH. Spatial regression models indicated that after adjustment for ecological factors, distance to the main study hospital was positively related to vaccine efficacy, increasing at a rate of 4.5% per kilometer distance. source
Ranges start at -486 effective. Negative efficacy if you live close to the hospital. what a joke.
From “A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children”
Collectively, they reported 469 VT IPD cases classified as vaccine failures and 403 as breakthrough. Vaccine failure and breakthrough rates were low: 8.4% and 9.3%, respectively, of all IPD in vaccinated children, consistent with the vaccines' high effectiveness. The main serotypes associated with vaccine failure/breakthrough were 19A, 3 and 19F for PCV13 and 14, 6B and vaccine-related 19A and 6A for PCV10.
Expert opinion: As we move to vaccines with more serotypes, it is not only important to consider which serotypes are added, but also monitor and address incomplete protection against specific serotypes.
During the study period, we recruited 188 patients 2–59 months of age who were admitted to the 3 participating centers with IPD. We identified the S. pneumoniae serotype causing IPD in 180 cases (95.7%), of which 104 (57.8%) were caused by PCV13 serotypes. Serotype 3 was the most frequent serotype (42 cases, 23.3%), followed by serotype 1 (19 cases, 10.6%), 19A (17 cases, 9.4%), and 14 (13 cases, 7.2%). Of the 180 case-patients, 102 (56.7%) were not vaccinated, 66 (36.6%) were age-appropriately vaccinated, and 12 (6.7%) were age-incorrectly vaccinated.
So its a very leaky vaccine that clearly does not cover all the serotypes.
Thirty-seven (34%) were in children who were completely vaccinated representing 13% of all IPD cases.
Basically everyone.
No consensus for a pre-defined threshold antibody level was reached. Affinity maturation may contribute to protection, but its role has not been established. source
Plotkin cited this study. He knows there is no threshold once again discrediting his own statement antibodies are responsible for clinical protection which it does not provide. And it is super safe according to the package insert.
serious adverse events up to 6 months following vaccination with the 4-dose series were reported by 9.6% of VAXNEUVANCE recipients and by 8.9% of Prevnar 13 recipients. Participants in these studies may have received either US-licensed or non-US licensed concomitant vaccines according to the local recommended schedule.
6. HEPATITIS B
From the CDC source:
Hepatitis B is a disease caused by the hepatitis B virus (HBV) that can be self-limited for some and lifelong for others. HBV is transmitted through the blood or bodily fluids of an infected person. In the United States, injection drug use (IDU) and having multiple sexual partners are the first and second most common risk behaviors or exposures reported for acute hepatitis B, respectively (3). Approximately 50–70% of people with acute hepatitis B are not symptomatic …
Approximately 90% of people >5 years of age with acute hepatitis B will spontaneously clear their infection (50, 51). People with resolved hepatitis B will remain positive for total anti-HBc and develop anti-HBs that protect against future HBV infection
Exchange of blood and bodily fluids and IV drug use? Are babies at risk of this if their mothers do not have it? There is only one reference for evidence (66).
“in The Gambia. Sera from 700 of a cohort of 1041 children vaccinated against hepatitis B in infancy were serially tested for markers of hepatitis B until age 7 years. No absolute level of protection against infection was found, but all children who attained a peak antibody response to vaccination of >=10 IU/L were protected against carriage of hepatitis B surface antigen”
First comment is the population. Children from the Gambia are not representative of the North American population. HBV infection prevalence is above 8% in The Gambia and 0.4% in the United States. They are not comparable. Note section 6 FDA Package Insert which makes the warning clinical trials may not reflect rates observed in practice for adverse reactions. Why should we assume the benefits will? Not surprisingly no absolute level of protection was found.
It should be mentioned the safety was only monitored for 4 days post administration. This is negligent and I agree with ICAN the Hep B shot should never have been allowed to be licensed based off these data. reference
58 newborns is an inadequate sample size. 2 became carriers so this is not a sterilizing injection. They are assuming a carrier rate to get their efficacy rate? This is not science.
5.8 Limitations of Vaccine Effectiveness Hepatitis B has a long incubation period. ENGERIX-B may not prevent hepatitis B infection in individuals who had an unrecognized hepatitis B infection at the time of vaccine administration. Additionally, it may not prevent infection in individuals who do not achieve protective antibody titers.
About 2–10% of healthy individuals fail to mount antibody levels to routine vaccines. source
and from “Non-responders to hepatitis B vaccination: a review” source
Of healthy vaccinees, 5-10% fail to mount an adequate antibody response. The antibody levels of an unknown further fraction of vaccinees fall considerably over time rendering them at a potential risk of infection.
and how long do these antibodies last? from “Duration of immunity after hepatitis B vaccination: efficacy of low-dose booster vaccine” source
We studied 245 hospital employees 3 years after primary vaccination with hepatitis B vaccine to determine the prevalence of immunity indicated by levels of antibody to hepatitis B surface antigen of 10 mIU/mL or greater; and to compare the immunogenicity of low-dose intradermal vaccine with standard-dose intramuscular vaccine in persons found to be seronegative. Thirty-eight percent of employees studied had antibody levels less than 10 mIU/mL. Low levels were associated with smoking, older age, and higher body-mass index. Seventy-eight percent of persons with low antibody levels responded to a single booster vaccine.
38% failure rate is a lot higher than the 5-10% previously quoted. They do not use preventing the clinical outcomes that patients would care about like preventing chronic infection, cirrhosis and hepatocellular carcinoma. Moving on.
7. ROTOVIRUS
From Plotkin’s paper:
Arguably, rotavirus vaccination provides the most complex and controversial puzzle with respect to definition of correlates of immunity in current vaccinology. Neutralizing antibodies, nonneutralizing antibodies, secretory antibodies, and cellular immune responses have all been proposed as correlates, and indeed, it may be that all of these play a role, depending on the situation.
I think their strategy is to make the water seem muddy to make it appear deeper than it really is. The want you to feel confused and that it is too complicated so people just give up.
From the package insert
So you do not know how it works? That being said Rotovirus actually used the primary clinical outcome of preventing rotavirus gastroenteritis and they report positive results.
And how well did it work in the real world?
Live, oral rotavirus vaccines are more effective at preventing rotavirus disease in countries with low child mortality compared with high child mortality. Among several hypotheses, poorer protection in malnourished children, who are more prevalent in countries with high child mortality, may partially explain this difference. We conducted a literature search to identify articles with a laboratory-confirmed rotavirus endpoint that evaluated differences by malnutrition status in rotavirus vaccine effectiveness and vaccine efficacy (VE) or the prevalence of rotavirus infection or illness among children <5 years old. We identified 7 analyses from 11 countries published from 2007 to 2019 that stratified rotavirus VE by malnutrition status. Among well-nourished children, VE point estimates ranged from 71% to 84% in observational studies and 26% to 61% in clinical trials. Among malnourished children, they ranged from -28% to 45% in observational studies and -3% to 61% in clinical trials. The relative difference between VE in well-nourished and malnourished children by length-for-age ranged from 37% to 64%, by weight-for-age ranged from 0% to 107%, and by weight-for-height ranged from -65% to 137%. We identified 3 cohort and 6 cross-sectional studies of natural rotavirus infection and illness and none reported that malnourished children were more susceptible to rotavirus infection or illness than well-nourished children. Overall, rotavirus vaccines may offer less protection to children with malnutrition than well-nourished children. source
So it does not work where its needed and unsurprisingly does not work in the malnourished. Maybe proper nutrition is more important than vaccination.
From “Effectiveness of Rotavirus Vaccination: A Systematic Review of the First Decade of Global Postlicensure Data, 2006–2016” source
Two rotavirus vaccines, Rotarix (RV1) and RotaTeq (RV5), were licensed for global use in 2006. A systematic review of 48 peer- reviewed articles with postlicensure data from 24 countries showed a median RV1 vaccine effectiveness (VE) of 84%, 75%, and 57% in countries with low, medium, and high child mortality, respectively, and RV5 VE of 90% and 45% in countries with low and high child mortality, respectively
There is no linking of antibodies to preventing rotovirus gastroenteritis so again the concept is inconsistent. The real world results are substantially lower than the clinical trial suggested. Also from the package insert it sheds and has a suspiciously high death rate:
Deaths: During the entire course of 8 clinical studies (Studies 1 to 8), there were 68 (0.19%) deaths following administration of ROTARIX (n = 36,755) and 50 (0.15%) deaths following placebo administration (n = 34,454). The most commonly reported cause of death following vaccination was pneumonia, which was observed in 19 (0.05%) recipients of ROTARIX and 10 (0.03%) placebo recipients (RR: 1.74, 95% CI: 0.76, 4.23).
Summary
Both the CDC and Stanley Plotkin repeat the unsubstantiated claim antibodies are responsible for preventing clinical infection. There is not a single example of an absolute correlate of protection. Plotkin cites references that do not attempt to experimentally and statistically test the threshold that an antibody provides sterilizing clinical protection. Vaccine failure occurs for all disease with any level of antibody titers. The author even attempts to argue that patients without seroprotection still have clinical protection which disproves it is antibodies that are responsible for the protection. This is false equivalence and skillful deception of language is intended to focus the patient on a meaningless surrogate so they take a product with no proven durable clinical benefit. Vaccines are a scam.







































Before the age of digital payments systems, paper cheques were not just used for payments, but sometimes used for a type of fraud called “floating cheques”. A deposit could be made for an amount which would credit one account before there was time to verify the funds and debit the other account. In the meantime, another empty cheque could be written from somewhere else to cover the incoming debit from the original cheque. The claims in vaccine mythology work much the same way. One paper cites another which cites another which cites another. It’s one situation where there is rarely someone who cares what is there to back up what is promised. Cronies.
Reading this was a pleasure 🙏